Over the past 15 years, we, as neurologists, have faced a profound dilemma when diagnosing Alzheimer’s disease, especially in light of the limited treatment options available for patients with Mild Cognitive Impairment (MCI) and dementia.
While we have consistently advocated and implemented comprehensive lifestyle changes for individuals in the early stages of cognitive decline and dementia, we have lacked evidence to suggest that these changes can halt or reverse the progression once Alzheimer’s disease has fully manifested.
Nonetheless, recent developments have instilled a glimmer of hope into our practice and the lives of our patients. Last week, we found out that the Phase 3 study of donanemab, an investigational therapy, has yielded encouraging outcomes, representing a notable advancement in the fight against Alzheimer’s disease.
Although it may not serve as a cure, the potential benefits of combining donanemab with lifestyle modifications can offer newfound hope to patients and their families.
Over the last few years, monoclonal antibody treatments — treatments which use lab-produced molecules engineered as substitutes for the antibodies our bodies naturally create — have emerged as a significant area of research in the pursuit of effective therapies for Alzheimer’s disease.
However, thus far, the results have been somewhat underwhelming. Before delving into these latest insights on monoclonal antibody treatments, it is essential to provide a brief overview of the history and current state of these drugs.
A brief history of monoclonal antibody treatments
Aducanumab (2019)
This drug gained attention for its potential to reduce amyloid plaques in the brain — a hallmark of Alzheimer’s disease. When abnormal levels of a protein called ‘beta-amyloid 42’ build up between neurons, regular cell function is disrupted in the brain. In 2019, aducanumab became the first monoclonal antibody to receive breakthrough therapy designation from the U.S. Food and Drug Administration (FDA). However, in 2021, the FDA issued a controversial decision regarding its approval, expressing concerns about conflicting trial results and the need for additional evidence to establish its effectiveness. Aducanumab’s current status as we write this in May 2023 remains under review, with ongoing discussions regarding its potential benefits and risks.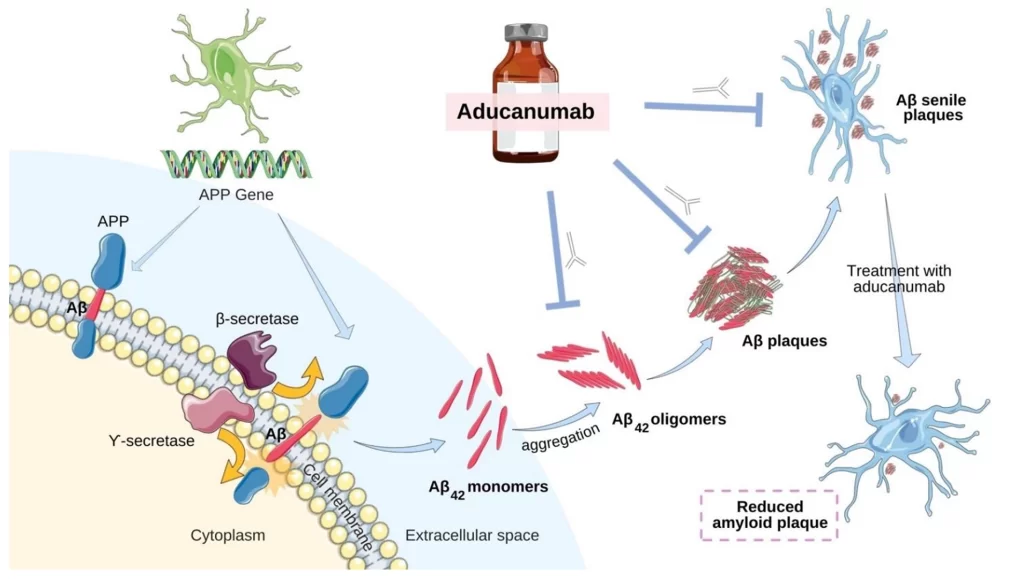
BAN2401 (2020)
BAN2401 is a monoclonal antibody that targets amyloid plaques. In a Phase 2 clinical trial, it showed positive outcomes by reducing amyloid levels and slowing cognitive decline. Unfortunately, BAN2401 has been associated with potential side effects such as brain swelling and inflammation, leading to cautious consideration of its risk-benefit profile. Further studies are ongoing to determine its long-term efficacy and safety.Donanemab (2021)
Donanemab is an investigational monoclonal antibody that selectively targets a modified form of beta-amyloid called N3pG. In a Phase 2 clinical trial, it demonstrated significant reduction in amyloid plaques and showed potential for improving cognitive decline. Donanemab has been generally well-tolerated, with side effects including ARIA (amyloid-related imaging abnormalities) observed in some patients. Further research and larger-scale trials are underway to assess its efficacy and safety for potential approval.Lecanemab (2021)
Lecanemab, formerly known as BAN2401, has shown promise in a Phase 3 clinical trial.
It demonstrated a reduction in cognitive decline and a decrease in amyloid plaques.
Similar to BAN2401, lecanemab has been associated with the risk of ARIA side effects. Ongoing research and regulatory evaluations are necessary to determine its long-term benefits and risks.
The third phase of donanemab trials
This new Phase 3 trial of donanemab may be a breakthrough in Alzheimer’s research.
The ‘TRAILBLAZER-ALZ 2’ study, conducted by Eli Lilly and Company, has captured the attention of the medical community with its groundbreaking findings.
This randomized, double-blind (both the participants and the researcher only find out which treatment the participants are receiving when the trial is finished), placebo-controlled (some participants receive a real treatment, whilst others get a ‘fake’ placebo) trial enrolled individuals with early symptomatic Alzheimer’s disease, providing a rigorous evaluation of donanemab’s effectiveness.
The primary endpoint of the study was the change in the integrated Alzheimer’s Disease Rating Scale (iADRS) from baseline to 18 months, measuring cognition and activities of daily living. The results were highly encouraging, as donanemab demonstrated a remarkable 35% slowing of cognitive and functional decline compared to the placebo group (p<0.0001).
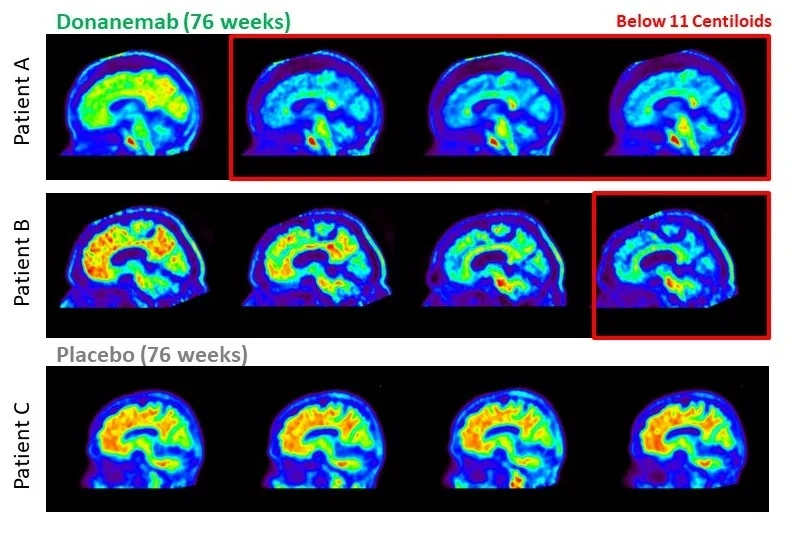
Reviving hope: key findings of the TRAILBLAZER-ALZ 2 study
The positive outcomes of the TRAILBLAZER-ALZ 2 study have reignited hope for patients and their families, offering a glimpse into a potential future where Alzheimer’s disease can be better managed.
Here are the key findings that highlight the significance of donanemab:
Slowing of clinical decline
Patients receiving donanemab experienced a notable slowing of clinical decline compared to those on placebo.
The ability to perform daily activities showed a 40% reduction in decline at 18 months (p<0.0001).
This finding holds immense promise for individuals in the early stages of Alzheimer’s, suggesting that donanemab may help them maintain their independence and engagement in meaningful activities for an extended period.
Reduction in disease severity
An essential measure of disease severity, the Clinical Dementia Rating-Sum of Boxes (CDR-SB), revealed an exciting outcome.
At one year, 47% of participants on donanemab exhibited no decline in CDR-SB, while only 29% of the placebo group achieved the same result (p<0.001). This finding suggests that donanemab could effectively slow the progression of Alzheimer’s disease, potentially enhancing the quality of life for patients.
Amyloid plaque clearance
Donanemab showcased significant reductions in brain amyloid plaque levels, as confirmed through amyloid positron emission tomography (PET) brain scans.
Within 6 months of treatment initiation, 34% of participants in the intermediate tau population achieved amyloid clearance, increasing to 71% at 12 months. This discovery is particularly significant as amyloid plaque is a defining characteristic of Alzheimer’s disease, and its removal may have a positive impact on clinical outcomes.
Safety and tolerability
While the benefits of donanemab are remarkable, it is crucial to consider the associated risks.
The most common side effect observed was amyloid-related imaging abnormalities (ARIA), which can manifest as temporary brain swelling or microhemorrhages.
Fortunately, the majority of ARIA cases were mild to moderate and resolved with appropriate medical management.
Nonetheless, ongoing research aims to optimize dosing strategies and minimize the occurrence of ARIA, ensuring that the benefits of donanemab outweigh any potential risks.
The importance of lifestyle changes: an holistic approach
While donanemab represents a significant advancement in Alzheimer’s research, it is crucial to emphasize the importance of a holistic approach to disease management.
As neurologists, we have witnessed the impact of lifestyle changes on cognitive health, even in the absence of curative treatments.
Implementing a comprehensive plan that includes physical exercise, mental stimulation, social engagement, and a healthy diet can complement the benefits of donanemab, leading to enhanced outcomes for patients.
Healthy diet
A nutritious diet rich in fruits, vegetables, whole grains, plant based proteins, and healthy fats is essential for brain health.
Diets like the Mediterranean, DASH (Dietary Approaches to Stop Hypertension), and Whole Food Plant Based (WFPB) have shown potential in reducing the risk of cognitive decline.
These diets emphasize anti-inflammatories, antioxidants, omega-3 fatty acids, and other nutrients that support brain function.
Physical exercise
Regular physical activity has been shown to improve cognitive function and reduce the risk of Alzheimer’s disease.
Encouraging patients to engage in activities such as brisk walking, swimming, or dancing can promote brain health and overall well-being.
Exercise increases blood flow to the brain, stimulates the growth of new neurons, and enhances memory and cognitive abilities.
Mental stimulation
Keeping the mind active through activities like puzzles, reading, learning new skills, or engaging in hobbies can help preserve cognitive function.
Mental stimulation challenges the brain, strengthens neural connections, and may contribute to a higher cognitive reserve, which can buffer against the effects of Alzheimer’s disease.
Social engagement
Maintaining social connections is vital for emotional well-being and cognitive health.
Encouraging patients to participate in social activities, join clubs, volunteer, or spend time with loved ones fosters a sense of belonging and provides opportunities for mental stimulation.
Social interaction can also reduce feelings of isolation and depression, which are common among individuals with Alzheimer’s disease.
Restorative sleep
Restorative sleep plays a vital role in disease management. As neurologists, we recognize its profound impact on cognitive health.
To promote restorative sleep, patients should establish consistent sleep patterns and adopt healthy sleep hygiene practices.
This includes creating a relaxing sleep environment, maintaining a regular sleep schedule, and avoiding stimulating activities before bedtime.
Engaging in relaxation techniques can also aid in improving sleep quality.
Avoiding cigarettes, alcohol
Smoking and heavy drinking increase the risk of cognitive decline and dementia. Encouraging patients to quit smoking and reduce alcohol intake can significantly reduce these risks.
The power of hope: a new era in Alzheimer’s care
Donanemab’s potential, when combined with lifestyle changes, offers a renewed sense of hope for patients and their families who have battled Alzheimer’s disease for years.
As neurologists, we understand the frustration of diagnosing individuals with early dementia while feeling limited in our ability to provide effective interventions.
However, the positive results from the TRAILBLAZER-ALZ 2 study remind us of the progress being made in Alzheimer’s research and the importance of staying optimistic.
Donanemab represents a breakthrough in the fight against Alzheimer’s, demonstrating its ability to slow cognitive and functional decline, reduce disease severity, and clear amyloid plaque.
While it may not be a cure, it opens doors to improved management strategies and enhanced quality of life for those affected by this devastating disease.
As we continue to navigate the complexities of Alzheimer’s care, we are committed to advocating for our patients and their families.
The combination of donanemab and lifestyle changes holds promise for a more comprehensive and holistic approach to managing Alzheimer’s disease.
It is crucial to empower individuals with knowledge about these advancements, offering them hope and reassurance that progress is being made.
The journey of the last 15 years as neurologists, diagnosing patients with MCI and dementia, has been filled with frustration and a longing for better treatment options.
However, the emergence of donanemab and its positive outcomes in the TRAILBLAZER-ALZ 2 study have injected hope into our practice and the lives of our patients.

Sources
- Pontecorvo, M. J., Lu, M., Burnham, S. C., Schade, A. E., Dage, J. L., Shcherbinin, S., … & Mintun, M. A. (2022). Association of donanemab treatment with exploratory plasma biomarkers in early symptomatic Alzheimer disease: a secondary analysis of the TRAILBLAZER-ALZ randomized clinical trial. JAMA neurology, 79(12), 1250-1259.
- Wessels, A. M., Dennehy, E. B., Dowsett, S. A., Dickson, S. P., & Hendrix, S. B. (2023). Meaningful Clinical Changes in Alzheimer Disease Measured With the iADRS and Illustrated Using the Donanemab TRAILBLAZER-ALZ Study Findings. Neurology: Clinical Practice, 13(2).
- Salloway, S., Farlow, M., McDade, E., & Tariot, P. (2021). A phase 2 multiple ascending dose trial of intravenous bapineuzumab in mild to moderate Alzheimer disease. Neurology, 76(18), 1545-1552. doi:10.1212/WNL.0b013e318217e7a4
- Mintun, M. A., Lo, A. C., Duggan Evans, C., Wessels, A. M., Ardayfio, P. A., Andersen, S. W., . . . Skovronsky, D. M. (2021). Donanemab in early Alzheimer’s disease. New England Journal of Medicine, 384(18), 1691-1704. doi:10.1056/NEJMoa2100708
- Sevigny, J., Chiao, P., Bussière, T., Weinreb, P. H., Williams, L., Maier, M., . . . Cummings, J. L. (2016). The antibody aducanumab reduces Aβ plaques in Alzheimer’s disease. Nature, 537(7618), 50-56. doi:10.1038/nature19323
- Honig, L. S., Vellas, B., Woodward, M., Boada, M., Bullock, R., Borrie, M., . . . Vellas, B. (2018). Trial of solanezumab for mild dementia due to Alzheimer’s disease. New England Journal of Medicine, 378(4), 321-330. doi:10.1056/NEJMoa1705971
- Mintun, M. A., Lo, A. C., Duggan Evans, C., Wessels, A. M., Ardayfio, P. A., aAndersen, S. W., . . . Skovronsky, D. M. (2021). Donanemab in early Alzheimer’s disease. New England Journal of Medicine, 384(18), 1691-1704. doi:10.1056/NEJMoa2100708



